Interleukin 12
IL-12, also termed IL-12p70, was independently discovered in 1989 by Kobayashi M. et al. (termed natural killer cell stimulatory factor) and in 1990 by Stern A.S. et al. (termed cytotoxic lymphocyte maturation factor).
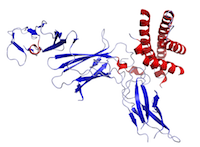
Figure This is a structure of IL-12 created using the data from Protein Data Bank (PDB: 1F45) and rendered using PyMOL.
IL-12 induces the differentiation of naive CD4+ T cells into Th1 cells and activates NK cells. Upon activation, NK cells produce IFN-γ and IL-2 but also IL-12 pointing to a positive feedback mechanism. IL-12 protects CD4+ Th1 cells from antigen-induced apoptotic death and was found to have synergistic effects with IL-18 in developing Th1 cells. In addition, IL-12 plays a role in T cell trafficking and migration by inducing functional adhesion molecules such as P- and E-selectin ligand expression on Th1 cells but not Th2 cells. Therefore, Th1 cells are selectively recruited to sites promoting a Th1 immune response. Functionally, these cells help in clearance of intracellular pathogens; in contrast to Th2 cells which are responsible for humoral immunity protecting against extracellular invaders. The balance between IL-12, favoring Th1 responses, and IL-4, favoring Th2 responses, determines the early preference expressed in the immune response.
IL-12p35 share substantial sequence homology to the cytokine IL-6 whereas the p40 chain is structurally homologous to the extracellular domain of the IL-6 receptor (IL-6R) α-chain. This suggests that the structure of IL-12 is evolved from a primordial cytokine of the IL-6 family and one of its receptors. Although IL-12p35 transcripts are found in many cell types, free IL-12p35 is not secreted without the IL-12p40 subunit. This latter subunit is produced predominantly by activated monocytes, macrophages, neutrophils, and dendritic cells. The biological activities of IL-12p70 are mediated via binding to a membrane receptor complex (IL-12R) composed of two subunits: IL-12R-β1 and IL-12R-β2. While the IL12Rβ1 subunit is constitutively expressed, the expression of IL-12R-β2 on Th1 cells is upregulated by IFN-γ and correlates with responsiveness to IL-12. Upon binding of IL-12, the JAK-STAT signaling pathway is activated, with STAT4 being the predominant mediator of T cell responses.
Detecting IL-12 with U-CyTech products
ELISA products
Human IL-12p70 ELISA
Old World Monkey IL-12/23p40 ELISA
New World Monkey IL-12/23p40 ELISA (marmoset)
ELISPOT products
Human IL-12p70 ELISPOT
Old World Monkey IL-12/23p40 ELISPOT