 Interleukin 6
Interleukin 6
The history of interleukin 6 (IL-6) dates back to the late 1960s when it was hypothesized that a T cell-derived soluble factor would be involved in B cell activation, which was initially named B cell stimulatory factor-2 (BSF-2). In 1986 the complementary DNA encoding this factor was cloned and the name IL-6 was introduced.
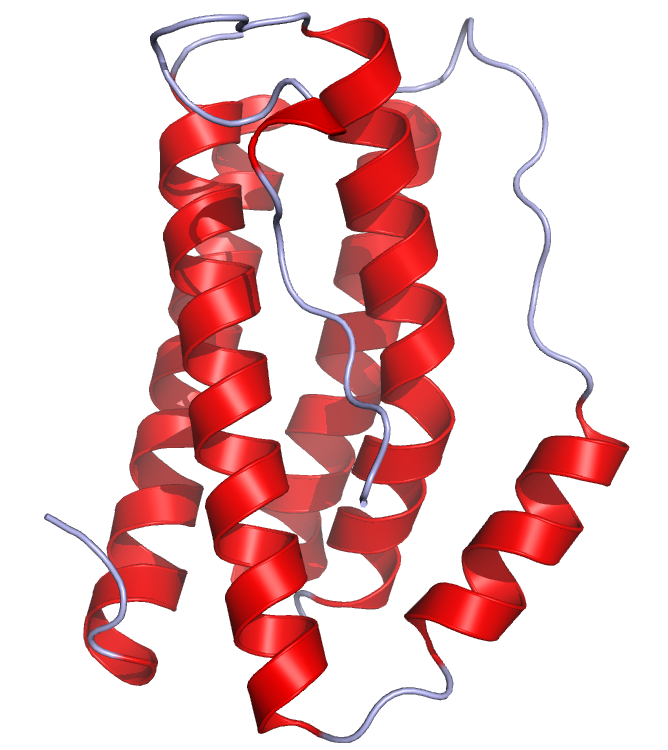
Figure This is a crystal structure of human IL-6 created using the data from Protein Data Bank (PDB: 1ALU) and rendered using PyMOL.
IL-6 (a glycosylated protein of 21–28 kDa) is produced by a broad spectrum of cells in response to a wide variety of stimuli. The cytokine is produced by innate immune cells (e.g., macrophages, dendritic cells, and mast cells), B cells, by some CD4 effector T helper (Th) cells and also by a variety of non-leukocyte cells (e.g. endothelial cells, fibroblasts, astrocytes, epithelial cells). A common feature of many of the stimuli that activate IL-6 is that they represent tissue damage or stress (e.g., UV, irradiation, reactive oxygen species, microbial products, viruses, or other pro-inflammatory cytokines). But IL-6 is also elevated in response to muscle contraction where it is thought to act in a hormone-like manner to augment substrate delivery.
While IL-6 has strong pro-inflammatory properties, IL-6 has also been reported to modulate the balance between Th1 and Th2 cells. IL-6 promotes the differentiation of Th2 cells by enhancing endogenous IL-4 and IL-13 production but diminishing IFN-γ signaling. In addition, IL-6 promotes the differentiation of Th17 cells in combination with transforming growth factor-beta (TGF-β) and blocks regulatory T (Treg) cell activity in vitro. As TGF-β is known to promote the generation of Treg cells, it has been proposed that IL-6 could fine-tune the balance between Treg cells and Th17 cells.
The pleiotropy and redundancy of IL-6 functions is explained by a unique receptor system comprising two functional proteins: a receptor specific for IL-6 (IL-6R) and gp130 the common signal transducer of many other cytokines. Two pathways mediate signal transduction via gp130: the JAK–STAT (Janus family tyrosine kinase–signal transducer and activator of transcription) pathway and the Ras–MAPK (mitogen-activated protein kinase) pathway. IL-6 first binds to the membrane-bound non-signaling IL-6R or to soluble free circulating IL-6R. Next, the IL-6/IL-6R complex binds to two molecules of gp130 and activates the cytoplasmic transcriptional factors, STAT1 and STAT3. Deregulation of IL-6 production plays critical roles in the pathogenesis of several disease processes, including human myeloma and several autoimmune diseases, such as rheumatoid arthritis, systemic-onset juvenile chronic arthritis (JCA), osteoporosis, and psoriasis.
Detecting IL-6 with U-CyTech products
ELISA products
Human IL-6 ELISA
Old World Monkey IL-6 ELISA
New World Monkey IL-6 ELISA (marmoset)
Mouse IL-6 ELISA
T cell ELISPOT products
Human IL-6 ELISPOT
Old World Monkey IL-6 ELISPOT
Mouse IL-6 ELISPOT