Interleukin 12
IL-12, also termed IL-12p70, was independently discovered in 1989 by Kobayashi et al. (termed natural killer cell stimulatory factor) and in 1991 by Gately et al. (termed cytotoxic lymphocyte maturation factor).
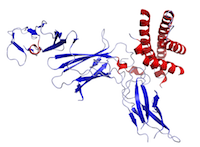
Figure This is a structure of IL-12 created using the data from Protein Data Bank (PDB: 1F45) and rendered using PyMOL.
IL-12 induces the differentiation of naive CD4+ T cells into Th1 cells and activates NK cells. Upon activation, NK cells produce IFN-γ and IL-2 but also IL-12 pointing to a positive feedback mechanism. IL-12 protects CD4+ Th1 cells from antigen-induced apoptotic death and was found to have synergistic effects with IL-18 in developing Th1 cells. In addition, IL-12 plays a role in T cell trafficking and migration by inducing functional adhesion molecules such as P- and E-selectin ligand expression on Th1 cells but not Th2 cells. Therefore, Th1 cells are selectively recruited to sites promoting a Th1 immune response. Functionally, these cells help in clearance of intracellular pathogens; in contrast to Th2 cells which are responsible for humoral immunity protecting against extracellular invaders. The balance between IL-12, favoring Th1 responses, and IL-4, favoring Th2 responses, determines the early preference expressed in the immune response.
IL-12p35 share substantial sequence homology to the cytokine IL-6 whereas the p40 chain is structurally homologous to the extracellular domain of the IL-6 receptor (IL-6R) α-chain. This suggests that the structure of IL-12 is evolved from a primordial cytokine of the IL-6 family and one of its receptors. Although IL-12p35 transcripts are found in many cell types, free IL-12p35 is not secreted without the IL-12p40 subunit. This latter subunit is produced predominantly by activated monocytes, macrophages, neutrophils, and dendritic cells. The biological activities of IL-12p70 are mediated via binding to a membrane receptor complex (IL-12R) composed of two subunits: IL-12R-β1 and IL-12R-β2. While the IL12Rβ1 subunit is constitutively expressed, the expression of IL-12R-β2 on Th1 cells is up regulated by IFN-γ and correlates with responsiveness to IL-12. Upon binding of IL-12, the JAK-STAT signaling pathway is activated, with STAT4 being the predominant mediator of T cell responses.
Detecting IL-12 with U-CyTech products
ELISA products
Human IL-12p70 ELISA
Old World Monkey IL-12/IL-23 p40 ELISA
New World Monkey IL-12/IL-23 p40 ELISA (marmoset)
ELISPOT products
Human IL-12p70 ELISPOT
Old World Monkey IL-12/23p40 ELISPOT